Batteries must be taken seriously, even if small. This applies especially to Li-ion.
The interest in batteries is growing, but stored energy must be respected. Children and adults alike are often unaware of the potential safety hazards when experimenting with batteries by heating cells, crushing them or applying an electrical short. Some batteries disintegrate with such a force they can cause serious harm, such as the loss of an arm or death. The damage inflicted depends on the chemistry and lithium-based cells are the most potent. Pay special attention to lithium-metal,the most energy-dense cell that is also the most powerful when stressed beyond its limits.
Health Canada reports receiving over 100 consumer reports in one year involving batteries. Failures involved overheating and starting fires. Officials responsible for safety say: “Any type of battery could be a potentially problem.” This applies mainly to batteries containing lithium. Smoke detectors are highly recommended to alert smoldering before a fire develops.
Folks are familiar with the alkaline, but this is the pussycat of batteries. Other systems are less forgiving and this article looks at common battery systems in the hands of the consumer. We learn how to care for batteries, what to watch for when traveling and how to stay out of trouble with correct handling.
Alkaline
|
Alkaline is the most common household battery. It provides longer runtime and is slightly more expensive than the zinc-carbon that it replaced. Lewis Urry (1927–2004) invented the alkaline in 1949 while working with the Eveready Battery Company laboratory in Ohio, USA.
A household alkaline has about 40 percent more energy than the average Li-ion. Alkaline is environmentally friendly and does not leak when depleted, as the old zinc-carbon did that came on the market in 1868. Alkaline has a very low self-discharge and can be stored for up to 10 years. It has a good safety record and can be carried on an aircraft without subject to UN Transport and other regulations.
The negative of alkaline is high internal resistance that limits current loading to light loads only, such as remote controls, flashlights and portable entertainment devices. This has an advantage in that alkaline can safely be carried in our jeans. An electrical short with keys or coins will cause the cell the heat up but there should be no fire.
|
|
|
 Figure 1: AA Alkaline cell
Figure 1: AA Alkaline cell
In spite of alkaline being forgiving, never mix batteries metallic objects. Do not store batteries in close proximity to flammable materials. There are reported incidents of batteries setting fire. Fire inspectors recommend storage containers that secure batteries so the positive and negatives terminals won’t touch.
Alkaline batteries cannot be charged, or at least they should not. This does not stop individuals from trying to get some energy back into the cell. Ordinary alkaline batteries have been recharged in households for many years. Recharging is most effective if the cell is not discharged beyond 50 percent. Depth of discharge governs the number of recharges and is limited to just a few. Battery makers do not endorse this practice for safety reasons; charging ordinary alkaline batteries may generate hydrogen gas that can lead to an explosion. (See BU-211: Alternate Battery Systems; go to Reusable Alkaline).
Button cells
The button cell, also known as a coin cell, is used in toys, watches, hearing aids and medical devices. Children like to play with button cells, putting them in their mouth and possibly swallowing them. In the United States alone, each year more than 2,800 children are treated in emergency rooms for swallowing button batteries. According to a 2015 report, injuries and deaths from swallowing batteries has increased nine-fold in the last decade. (See BU-703: Health Concerns with Batteries)
|
If swallowed, the battery often gets stuck in the esophagus (the tube that passes food). Water or saliva creates a conduit for electrical current that triggers a chemical reaction producing hydroxide, a caustic ion that can cause serious burns to the surrounding tissue. Doctors often misdiagnose the revealing symptoms revealing as fever, vomiting, poor appetite and weariness. Batteries that make it through the esophagus often move through the digestive tract with little or no lasting damage, although they can ulcerate the stomach wall.
The advice to parents is to choose safe toys and to keep small batteries away from young children. Similar to pharmaceutical products, keep small batteries locked away from small children.
 Figure 2: Button or coin cells Figure 2: Button or coin cells
|
|
|
Lead acid
|
Lead acid produces high load current for a few seconds, causes sparks and melts metals but the battery soon gets exhausted. An analogy is a drying felt pen that works for short markings on paper and then needs resting to replenish the ink. While recovery is fast, charging is notoriously slow and gets worse with age. An electrical short can cause a spill and create exhaust gases. Do not try to short lead acid.
Lead is a toxic metal that can enter the body by inhalation of lead dust or ingestion when touching the mouth with lead-contaminated hands. Children and fetuses of pregnant women are most vulnerable to lead poisoning. Excessive levels of lead may affect a child’s growth, cause brain damage, harm kidneys, impair hearing and induce behavioral problems. In adults, lead can cause memory loss and lower the ability to concentrate, as well as harm the reproductive system. Lead is also known to induce high blood pressure, nerve disorders, and muscle and joint pain. Researchers think that Ludwig van Beethoven became ill and died because of lead poisoning.
 Figure 3: Sealed lead acid cell Figure 3: Sealed lead acid cell
|
|
|
Do not store starter batteries where children play as the terminals of a starter battery are made of lead. Lead acid also has one of the most corrosive electrolytes of all batteries that must be safeguarded.
As with all potentially dangerous items, the golden rule is “if unsure – seek professional advice”
The following is a non-exhaustive list of good practices when handling secondary batteries.
- Consult your vehicle, equipment and battery owner’s manuals for instructions and safety precautions
- Wear approved safety glasses or goggles.
- Wear proper clothing to protect your hands and body.
- Make sure the work area is well-ventilated.
- Remove all metal jewellery before working on or near a battery and never allow metal tools or vehicle components to come into contact with the battery terminals
- Never lean over a battery while boosting, testing or charging.
- Keep away from cigarettes, flames, sparks and other ignition sources – they can cause a battery to catch fire or explode.
- Always shield eyes and face from a battery.
- Do not charge or use booster cables or adjust post connections without proper instructions and training.
- Do not allow battery electrolyte to come into contact with fabrics or painted surfaces. If battery electrolyte comes into contact with any surface, the surface should be washed down immediately with copious amounts of clean water.
- KEEP BATTERIES LEVEL. DO NOT TILT.
- Handles on batteries are for positioning only and not for carrying.
- In event of an accident, flush with water and get medical assistance immediately.
- KEEP OUT OF REACH OF CHILDREN
Battery Electrolyte contains sulphuric acid, which cause burns to skin and eyes. We suggest you wear suitable eye protection and gloves when handling batteries.
Lithium-ion
|
The damage a lithium-ion cell can inflict by an accidental short comes at a surprise to most. Packaged in an 18650 cell (pictured) Li-ion looks like a harmless AA alkaline cell. Not so. When shorted, Li-ion delivers persistent current without abiding. This often leads to venting with flame, a violent self-destruction alike a rocket. Embedded safety components offer protection, but not all cells have such safeguards.
Many 18650 cells are carried as spares for vaping devices. E-cigarettes require high current to activate the heat element and only Li-ion has such a load capability. When carrying a spare, wrap battery in a plastic bag to prevent electrical short.
Because of potential danger, lithium batteries can no longer be placed in checked baggage, but must be carried onboard an aircraft. Quick access to a fire extinguisher enables putting out a fire in the cabin. Each passenger can take two spares Li-ion that do not exceed 160Wh each; 320Wh total. An 18650 rated at 3,300mAh has about 12Wh. (See BU-704a: Shipping Lithium-based Batteries by Air).
 Figure 4: Lithium-ion cell in 18650 format Figure 4: Lithium-ion cell in 18650 format
|
|
|
From 1991-2016, 138 airport and flight incidents involving lithium batteries occurred. These include 13 E-cigarettes, 7 mobile phones/tablets, 7 spare batteries and 4 laptops. E-cigarette incidents increased the most.
According to published reports, 68 percent of battery failure in transit is caused by short circuit, and poor packaging is much to blame. Do not store and transport bare batteries in a metal box; do not mix batteries with coins, house keys in your jeans. Always put cells and batteries in clear plastic bags.

 Figure 5: Li-ion cell burns jeans
Figure 5: Li-ion cell burns jeans
Figure 5 shows a pair of burned jeans in which a Li-ion cell came in contact with loose coins in the pocket. It inflicted third degree burns on the man’s leg. Little can be done to stop the flaming event once it has begun.
Lithium Batteries
While Li-ion is rechargeable, there are non-rechargeable lithium batteries that exceed Li-ion in capacity. They contain a metallic anode and are often called lithium-metal. This increases volatility and these batteries are subject to tighter shipping regulations than the more benign lithium-ion. Primary lithium batteries come in many varieties and are mainly used for industrial uses. (See BU-106a: Choices of Primary Batteries.)
Lithium iron disulfide (LiFeS2) is a replacement of the regular household alkaline with longer runtimes and better loading capabilities. Lithium batteries normally deliver 3 volts and higher, LiFeS2 is 1.5 volts, making it compatible with the AA and AAA formats. They cost slightly more than alkaline and must follow transportation rules.
Lithium thionyl chloride (LiSOCI2 or LTC) can withstand high heat and strong vibration. Thanks to the wide temperature operating range, LTC batteries are mainly used for horizontal drilling, also known as fracking that reaches head temperatures of 125°C (257°F). With a specific energy of over 500Wh/kg, they offer twice the capacity of the best Li-ion. Even though rugged and seemingly indestructible, this battery is one of the most potent if abused. Because of the potential danger, LTC is not permitted in consumer products; it also requires training for handling.
Lithium manganese dioxide (LiMnO2 or Li-M) is another common metallic lithium battery that is more benign than LTC. It comes with a lower capacity and is safe for public use. Typical uses are meter sensing, medical devices, road toll sensors and cameras.
Effect of Ageing
Li-ion batteries are safe but what may not be fully understood with a growing battery population is aging. Users ask: “Will my battery die quietly or depart with a bang?” Typical usage patterns that stress a Li-ion battery are excessive loading, rapid-charging and charging below freezing. Furthermore, storing Li-ion at a voltage below 2 volts per cell leads to dendrite growth that can damage the separator and cause a mild electrical short that can progress into a full electrical short, even if kept in storage. A small water leak in a faulty hydro dam can advance to a torrent and take a structure down, so also can separator damage lead to venting with flame. The temperature rises to 500°C (932°F), at which point the cell catches fire or explodes.
If a Li-ion battery overheats, hisses, or bulges, immediately move the device with battery away from flammable materials and place it on a non-combustible surface. If at all possible, remove the battery from the device and put it outdoors to burn out.
A small Li-ion fire can be handled like any other combustible fire. For best result use a foam extinguisher, CO2, ABC dry chemical, powdered graphite, copper powder or soda (sodium carbonate). If the fire occurs in an airplane cabin, the FAA instructs flight attendants to use water or soda pop. Water-based products are most readily available and are appropriate since Li-ion contains very little lithium metal that could react with water. Water also cools the adjacent area and prevents the fire from spreading. (See BU-304a: Safety Concerns with Li-ion).
A large Li-ion fire, such as in an EV, may need to burn out as water is ineffective. When encountering a fire with a lithium-metal battery, only use a Class D fire extinguisher. Lithium-metal contains of lithium that reacts with water and makes the fire worse. Do not use the Class D fire extinguisher for regular fires.
Statistics
A recent study from George Mason University estimated over 2,000 visits to U.S. emergency rooms caused from explosion-related injuries from e-cigarette burns from 2015 to 2017. Most injured were men putting e-cigarette batteries in their jeans or shirt pockets. Some also had keys in their pocket, a dangerous mix of metal and lithium-ion batteries causing an electric short. Many experienced severe burns to their legs, arms and hands, even death. Figure 6 provides statistics.
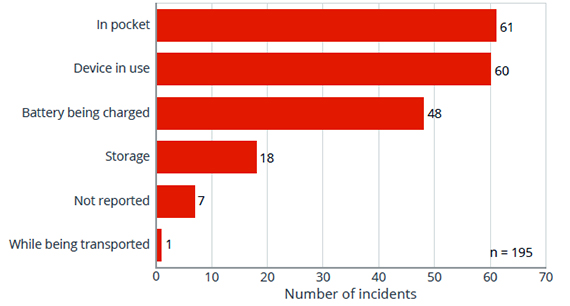
Figure 6: Statistics of battery incidents relating to e-cigarettes
Source: FEMA (U.S. Fire Administration)